Background: Treatment options for older, less fit acute myeloid leukemia (AML) patients continue to be challenging, with toxicities and limited efficacy. The combination therapy of azacitidine (Aza) and the bcl-2 inhibitor venetoclax (Ven) showed superior benefit in response rates and overall survival compared to Aza alone, leading to the combination's FDA approval in newly diagnosed AML patients ≥ 75 years and younger patients ineligible for intensive chemotherapy. While Ven-Aza is a very effective and potent therapy, the 28-day continuous dosing of Ven combined with 7 days of Aza resulted in interrupted dosing or shortened dosing schedules due to persistent cytopenias in more than 50% of treated patients in the VIALE-A trial and subsequent real-world data. Furthermore, investigation of novel agents in combination with Ven-Aza has been challenging due to prolonged cytopenias even when monotherapy with the new agent shows no myelosuppression. Given that Ven-Aza is not curative, successful integration of other agents with this standard of care remains important in AML. The growing knowledge of Ven-Aza toxicities prompted attention to developing a safer dosing regimen while maintaining similar efficacy. Our study will examine the FDA label of 28 days of Ven, versus 14 days, in combination with Aza for newly diagnosed AML patients ≥ 60 years who are not candidates for intensive chemotherapy. The primary objective of this study is to compare complete remission (CR) rates with an abbreviated schedule of Ven (14 days) versus the current package insert approved schedule (28 days). We hypothesize that reducing the dosing duration of Ven would reduce the associated toxicities while maintaining a comparable response rate.
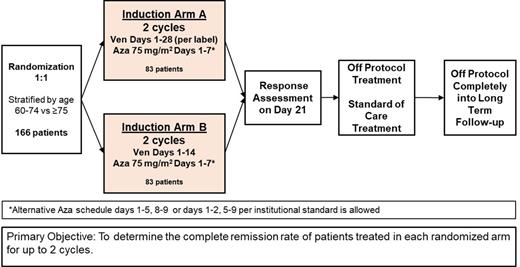
Study Design and Methods: This protocol is a sub-study of the Beat AML Master Trial (NCT03013998) in which untreated AML patients age ≥ 60 are assigned an investigational therapy based on cytogenetic and central genomic analysis. Patients are randomized onto Arm A (28-day Ven-Aza schedule) or Arm B (14-day Ven-Aza schedule) stratified by age (60-74 vs. 75+ years). The primary objective is to determine the complete remission (CR) rate of patients treated in each randomized arm for up to 2 cycles. The secondary objectives will include assessments of composite complete remission (CR, CR with hematologic improvement, CR with incomplete count recovery), duration of remission, survival, and incidence of treatment-related and non-related toxicities. Additionally, the overall incidence of febrile neutropenia, grade ≥3 infections, transfusions, days of hospitalization and time to neutrophil recovery will be determined.
In both arms of the study, patients will be assessed for response at day 21-28 of cycles 1 and 2. Patients who have marrow remission (< 5% blasts) will wait for count recovery of absolute neutrophil count ≥ 0.5x10 9/L and platelets ≥ 50x10 9/L before initiating the next cycle of therapy. Patients will then be followed for survival. With the exception of the Ven dosing schedule (14 vs. 28 days), the FDA approved package insert for Ven, including dose modifications for concurrent azole therapy, will be followed in both arms.
The intent of the analysis is to demonstrate that the 14-day Ven-Aza regimen is at least as effective as the standard 28-day regimen. A total of 166 patients (83 per group) will provide 80% power to detect a 10% non-inferiority margin at the one-sided 5% significance level, assuming that the CR rate is 36% for the 28-day regimen, as observed in VIALE-A, and 25% higher (45%) for the 14-day regimen. In addition to assessing the frequency of adverse events such as cytopenias, safety monitoring will include an assessment of response rates after at least 25% of patients have completed 2 cycles of treatment, with consideration for stopping the study if data suggest the 14-day regimen is inferior to the standard regimen. Correlative studies will include measurement of MRD and a focus on identifying properties of leukemic cells that respond, do not respond and become resistant to therapy.
OffLabel Disclosure:
Borate:RUNX1 Foundation: Honoraria; Servier: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Pfizer: Other: Research; Jazz: Other: Research; Incyte: Other; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Research. Zeidner:Gilead: Consultancy, Honoraria, Research Funding; Immunogen: Honoraria; Merck: Research Funding; Jazz: Research Funding; Shattuck Labs: Honoraria, Research Funding; Stemline: Research Funding; Sumitomo Dainippon Pharma: Research Funding; Takeda: Research Funding; Novartis: Consultancy; Servier: Consultancy, Honoraria; Sellas: Consultancy; Foghorn: Consultancy; Daiichi Sankyo: Honoraria; Astex: Research Funding; Arog: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Swords:Kronos Bio: Research Funding. Stein:Ono Pharma: Consultancy; Blueprint: Consultancy; OnCusp: Consultancy; Syndax: Consultancy; Gilead: Consultancy; Neoleukin: Consultancy; Abbvie: Consultancy; Genesis: Consultancy; Genentech: Consultancy; Menarini: Consultancy; Jazz: Consultancy; Agios: Consultancy; Janssen: Consultancy; PinotBio: Consultancy; Novartis: Consultancy; Bristol Myers Squib: Consultancy, Research Funding; Eisai: Research Funding; CTI Biopharma: Consultancy; Foghorn: Consultancy; Servier: Consultancy; Calithera: Consultancy; Daiichi: Consultancy; Aptose: Consultancy; Syros: Consultancy; Astellas: Consultancy. Baer:Takeda (Inst): Research Funding; FORMA Therapeutics (Inst): Research Funding; Kite, a Gilead company (Inst): Research Funding; Kura Oncology (Inst): Research Funding; Abbvie (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding. Stock:Servier: Other: Data Safety Monitoring Board/Advisory Board; Newave: Honoraria; Kura: Research Funding; Amgen: Honoraria; Glaxo Smith Kline: Consultancy; Kite: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria. Madanat:Rigel Pharmaceuticals: Honoraria; MD Education: Honoraria; OncLive: Honoraria; Novartis: Honoraria; Taiho oncology: Honoraria; Stemline therapeutics: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Sierra Oncology: Honoraria; GERON: Consultancy; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement. Olin:Astellas: Consultancy; Abbvie: Consultancy; Rigel: Consultancy; Servier: Consultancy; Cellectis: Research Funding; Actinium: Consultancy. Schiller:Stemline Therapeutics: Speakers Bureau; Sanofi: Research Funding, Speakers Bureau; Karyopharm Therapeutics: Research Funding, Speakers Bureau; Actinium Pharmaceuticals: Research Funding; Actuate Therapeutics: Research Funding; Arog: Research Funding; Celator: Research Funding; Constellation Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding; Deciphera: Research Funding; Delta-Fly Pharma: Research Funding; FORMA Therapeutics: Research Funding; Fujifilm: Research Funding; Gamida Cell: Research Funding; Genentech/Roche: Research Funding; Geron: Research Funding; Mateon Therapeutics: Research Funding; Onconova Therapeutics: Research Funding; Pfizer: Research Funding; Precog: Research Funding; REGiMMUNE: Research Funding; Samus Therapeutics: Research Funding; Sangamo Bioscience: Research Funding; Sellas Life Sciences: Research Funding; Stemline Therapeutics: Research Funding; Takeda: Research Funding; Tolero Pharmaceuticals: Research Funding; Trovagene: Research Funding; Agios: Research Funding; ElevateBio: Research Funding; Ono Pharmaceutical: Research Funding; AVM Biotechnology: Research Funding; Syros Pharmaceuticals: Research Funding; Kronos Bio: Research Funding; Kite: Research Funding, Speakers Bureau; Astellas Pharma: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding; Agios: Consultancy; Ono Pharmaceutical: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Amgen: Current equity holder in publicly-traded company, Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Research Funding, Speakers Bureau. Lin:Bio-path Holdings: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Celyad: Research Funding; Aptevo Therapeutics: Research Funding; Cleave Biosciences: Research Funding; Ciclomed: Research Funding; Jazz Pharmaceuticals: Research Funding. Curran:Amgen: Other: Advisory board; Kite: Other: Advisory board; Incyte: Other: Advisory board; Pfizer: Honoraria, Other: Advisory board; Jazz: Other: Advisory board; Servier: Consultancy, Other: Expert consensus panel. Levine:Zentalis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Auron: Membership on an entity's Board of Directors or advisory committees; Prelude: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Qiagen: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; AstraZeneca: Consultancy, Honoraria; Novartis: Consultancy; Roche: Honoraria; Lilly: Honoraria; Amgen: Honoraria; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Ajax: Membership on an entity's Board of Directors or advisory committees, Research Funding. Druker:US Patent and Trademark Office: Patents & Royalties: Patents 6958335 (Novartis exclusive license), 4326534, 7416873, 7592142, 10473667, 10664967, 11049247; Syndax: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Gilead: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Tolero: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Incyte: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Astellas: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Celgene: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Bristol Myers Squibb: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Dana-Farber Cancer Institute: Patents & Royalties: #2063 (licensed exclusively to Merck & Co); and #2524, Research Funding; DNA SEQ: Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Current Employment, Patents & Royalties: #1518 (exclusive option agreement with CytoImage); #0606/Patent 6958335 (Novartis exclusive license); #2573; #0843; #0996; AstraZeneca: Other: PI or Co-investigator on clinical trial(s) funded via contract with OHSU.; Enliven Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: PI or Co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; CureOne: Membership on an entity's Board of Directors or advisory committees; Therapy Architects, LLC: Membership on an entity's Board of Directors or advisory committees; Aileron Therapeutics: Membership on an entity's Board of Directors or advisory committees; Multicancer Early Detection (MCED) Consortium: Membership on an entity's Board of Directors or advisory committees; Beat AML LLC: Membership on an entity's Board of Directors or advisory committees; Vincerx Pharma, Inc.: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; VB Therapeutics: Membership on an entity's Board of Directors or advisory committees; The RUNX1 Research Foundation: Membership on an entity's Board of Directors or advisory committees; Recludix Pharma, Inc.: Consultancy, Current holder of stock options in a privately-held company; Labcorp: Membership on an entity's Board of Directors or advisory committees; Nemucore Medical Innovations, Inc.: Membership on an entity's Board of Directors or advisory committees; Iterion Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; GRAIL: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Cepheid: Membership on an entity's Board of Directors or advisory committees; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Adela, Inc.: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Amgen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Burd:Eilean Theraputics: Current Employment, Current equity holder in private company. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Byrd:Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES.
Venetoclax and Azacitidine for the treatment of AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal